A Deeper Look Inside: Retinol
The term “vitamin A”describes the molecule all-trans-retinol(Retinol). More loosely applied, the term is used to refer to set of compounds closely related to Retinol. Collectively Retinol and its derivatives are known as “retinoids”, a term that can be applied to natural or synthetic molecules with vitamin A activity.
Retinoids are required for maintaining many essential physiological processes in the body, including normal growth and development, normal vision, a healthy immune system, normal reproduction, regulation of inflammation, healthy skin and barrier functions and many others.
Vitamin A is an essential micronutrient for humans, meaning that it cannot be biosynthesized in the body but must be obtained from dietary sources. Vitamin A exists in the plant world only in the form of precursor compounds, such as β-carotene. This is a member of a large class of naturally occurring carotenoids, with about 50 compounds with vitamin A activity. We can also obtain vitamin A from animal sources in the form of retinyl esters.
Retinol and retinyl esters are the most abundant retinoid forms present in the body.
The most abundant retinyl esters present in the body are those of palmitic acid, oleic acid, stearic acid, and linoleic acid. The concentration of retinoic acid, the biologically active form of vitamin A, within tissues is generally very low and usually 100 to 1,000 times less than that of retinol. More than 90% of the body’s supply of vitamin A is stored in the liver.
Most of the absorbed vitamin A is transported from the small intestine to the liver, where it is stored predominantly as retinyl palmitate. It is released into the bloodstream in combination with a specific binding protein, from which target cells (such as skin cells) throughout the body take it up.
Although the liver and intestine are the major tissue sites of retinol esterification in the body, many tissues are able to esterify retinol and accumulate some retinyl ester stores, including the skin, eye, lung, adipose tissue, and spleen.
Metabolism of retinol
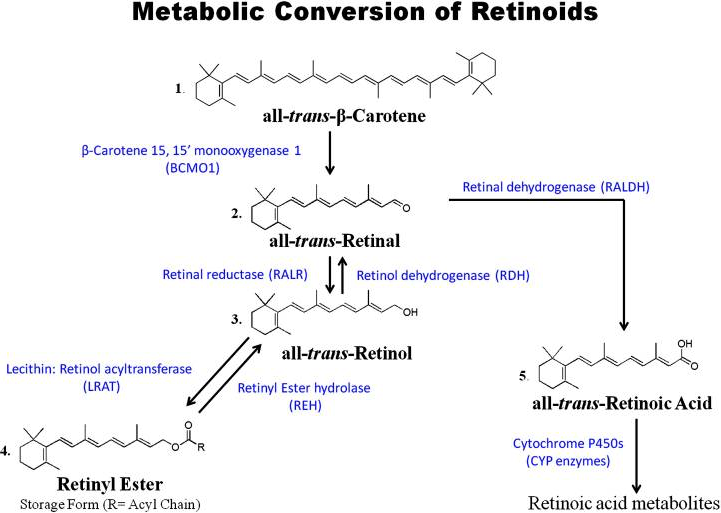
Retinol is mobilized from the liver by hydrolysis of retinyl esters; this process is interchangeable. Retinol is then oxidized reversibly to retinaldehyde (retinal), and then retinaldehyde is oxidized irreversibly to retinoic acid. Since there are no known enzymes that can convert retinoic acid back to retinal, excessive or unneeded retinoic acid is not recycled back to retinol/retinyl ester and must be catabolized and eliminated from the body.
Retinoic acid was identified as the biologically active form of vitamin A almost 70 years ago. For almost all biological functions that involve retinoids, from cellular differentiation to growth and development of organisms, sequential oxidation of retinol to retinoic acid must be achieved.
The retinoic acid status of cells is an important determinant of retinol metabolism. A cell deficient in retinoic acid oxidizes retinol to retinoic acid.
On the other hand, if a cell is sufficiently supplied with retinoic acid, retinol is esterified with fatty acids and stored intracellularly in the form of retinyl esters.
Within cells, retinol links with cellular retinol-binding protein. Research has shown that when retinol is topically applied to human skin, this retinol-binding protein and the level of retinyl esters noticeably increases. This reaction is likely a compensatory cellular response to an excess of retinol, a mechanism to promote storage of retinol as retinyl esters.
Is the whole-body economy of vitamin A an integrated, centrally regulated system?
The sequential oxidation of retinol to retinal (retinaldehyde) and then to retinoic acid is a very tightly regulated metabolic pathway.
Research shows no detectable increase in the level of retinoic acid in skin cells following topical application of retinol to skin.
The research further indicates that internally synthesized retinoic acid is much more effective than externally supplied retinoic acid in activating retinoid pathways in human skin.
This retinoic acid-responsive regulation is proposed to give rise to a positive feedback loop when cellular retinoic acid levels are high, the synthesis of retinyl esters is increased, preventing the creation of additional retinoic acid.
Various studies have shown that each molecule of retinol circulates several times a day between plasma and liver before undergoing irreversible disposal. Known as retinol recycling, this process seems to provide an ideal means for the liver to constantly sample and adjust the concentration of retinol available in plasma for peripheral tissues.
Vitamin or a hormone?
Both actually. Retinoic acid acts in a hormone like manner in that the nuclei of many types of cells contain at least three different proteins that serve as retinoic acid receptors. Once retinoic acid binds with its receptor, the complex changes its shape to regulate gene expression within the cell. Among the genes with which it interacts are those involved with the formation of the extracellular matrix (ECM) important for normal development of cells. Proteins within the cells serve to bind retinol or retinoic acid to regulate their concentration within the cells to prevent toxicity and to direct vitamin A molecules to sites where they are metabolised.
To sum up, we could say retinol is the main circulating form of the vitamin A, retinyl ester is the storage form and retinoic acid is the main active metabolite. The complex biochemical reactions within our bodies involving many enzymes and proteins work constantly to keep our bodies in balance (homeostasis) and ensuring excess retinoic acid is excreted, circulating retinol that is not needed is converted back to retinyl esters for storage and then re-converted back to retinol when needed.
Sources:
- Pharmacology and molecular mechanisms of retinoid action in skin – Author: S. Kang, G. J. Fisher, J. J. Voorhees, available: springer.com
- Retinoids: a journey from the molecular structures and mechanisms of action to clinical uses in dermatology and adverse effects, available: researchgate.net
- Vitamin A metabolism: An update, available: ncbi.nlm.nih.gov
- Retinol and retinyl esters: Biochemistry and Physiology, Author: Sheila M. O Byrne and William S. Blaner , Department of Medicine, College of Physicians and Surgeons, Columbia University
- Retinoids: a journey from the molecular structures and mechanisms of action to
- clinical uses in dermatology and adverse effects
- The biological significance of vitamin A in humans: A review of nutritional aspects and clinical considerations, Author: Ramadhan Oruch, Ian Pryme
- Vitamin A and Retinoids: An update of biological aspects and clinical applications, Edited by: M.A. Livrea





